The NCC is a body that protects consumers by ensuring that businesses comply with consumer protection laws and treat customers fairly.
The National Consumer Commission (NCC) has issued a product recall of certain Boston Scientific Encore medical devices and kits due to the discovery of foreign material particles in them.
There are 103 affected devices, and health facilities are urged to stop using them.
The NCC is a body that protects consumers by ensuring that businesses comply with consumer protection laws and treat customers fairly.
The Consumer Protection Act 68 of 2008 safeguards consumers from hazards and ensures their well-being and safety by governing product recalls. NCC can order a recall if a product is deemed unsafe or poses a potential risk to the public.
ALSO READ: These VW Polo Sedans and Tarmak basketball hoops are being recalled
Recall of medical devices
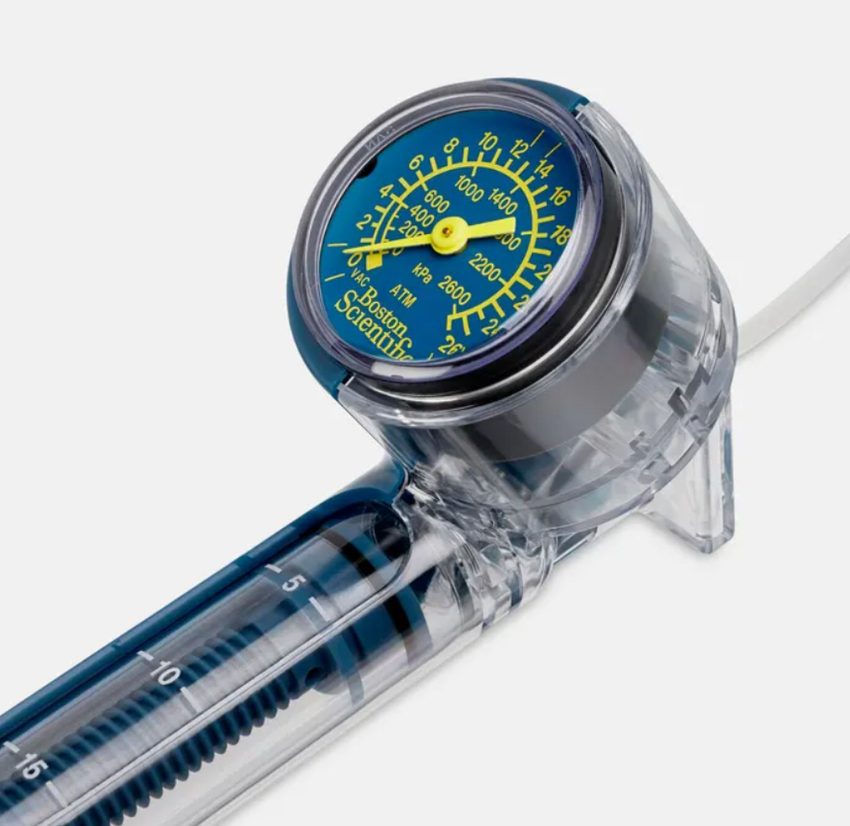
The NCC said Boston Scientific is recalling Encore26 Inflation Device, Encore26 Advantage Kits, NephroMax Kits and UroMax Ultra Kits made available from 28 February 2025.
“The Encore 26 Inflation Device (including Kits) is used with balloon dilation catheters to create and monitor pressure in the balloon and to deflate it.
“According to the manufacturer, the device is used in a variety of clinical applications, including interventional cardiology, neurovascular, endoscopy, urology, and peripheral vascular procedures.”
Issue with medical devices
Boston Scientific said they are issuing the recall because they discovered foreign material particles in the affected devices.
“During use, these particles could migrate from the affected devices into a balloon dilation catheter, impacting the ability to inflate or deflate the balloon used with the Encore 26 inflation device.
“The inability to inflate may lead to a prolongation of the procedure, while deflation issues may require the balloon to be ruptured so that it can be removed from the patient.”
ALSO READ: Product recall: Toys from Takealot, Toys R Us and e-bike batteries pulled over choking, fire hazards
Discontinue use
Boston Scientific has urged health facilities that use the medical devices to stop using them and segregate the affected product. The company will collect the defective devices.
The NCC over the past weeks have been known for issuing product recalls of various cars, with the latest being a Lexus LX600 due to an issue that can greatly increase the risk of an accident, especially at higher speeds.
Lexus South Africa told the NCC that the impacted cars are equipped with a V35A engine that contains crankshaft main bearings, which allow the crankshaft to rotate within the engine assembly while running.
Mass Ford recall
Ford Motor Company Southern Africa (FMCSA) issued a recall in late July affecting four models, totalling 5 718 cars that might be affected by the issues.
FMCSA stated that it is recalling EcoSports made between April 2021 and July 2022, Pumas from November 2021 to September 2024, and Rangers, as well as Everests, between June 2022 and March this year.
Ford said the cars were not only sold in South Africa, but in other parts of the Southern African Development Community (SADC) nations.
The recall of the EcoSport consists of 2 806 units in South Africa, 25 in Botswana and 41 in Namibia over front half shafts.
Dearborn states that the front half shafts could have been inserted improperly into the transmission.
In the case of the Puma, there are 1 775 cars affected in South Africa, six in Botswana, 13 in Namibia, and two in Eswatini.
NOW READ: Here’s why VW is recalling 253 Polo sedans – Is yours included?